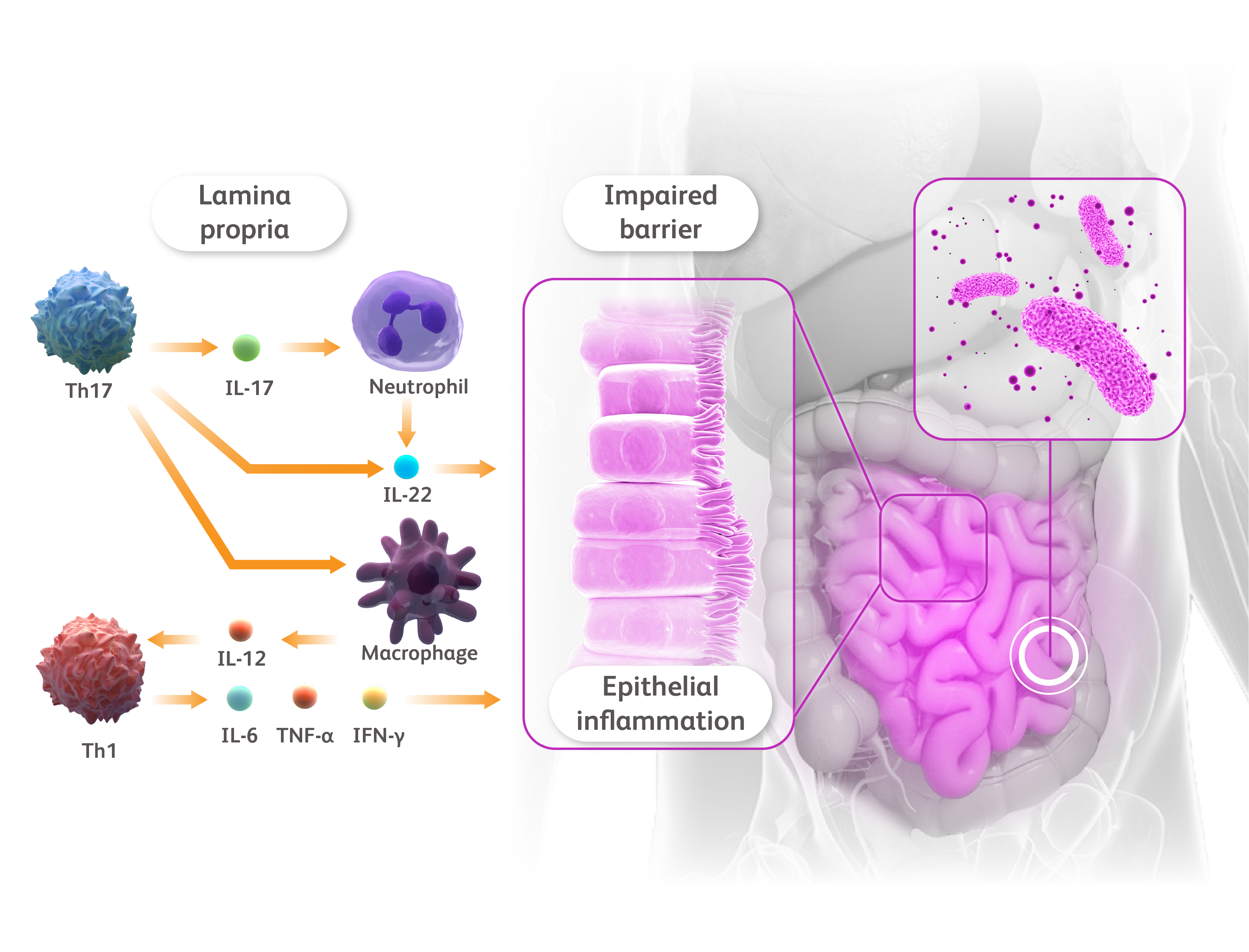
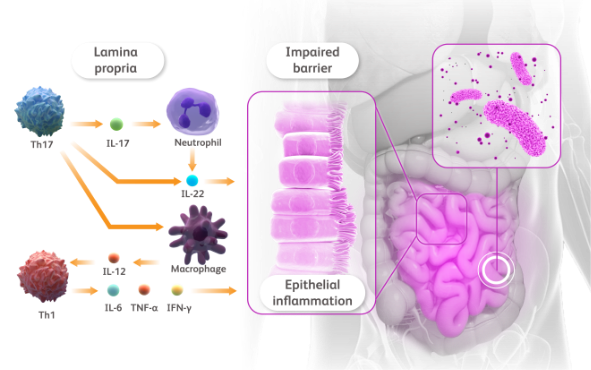
more than a skin disease
References
- Fu Y et al. JAMA Dermatol. 2018;154:1417–1423.
- Yao D et al. Inflamm Bowel Dis. 2019;25:1595–1602.
- Schmitt H et al. Semin Immunopathol. 2019;41:737–746.
- Elmets CA et al. J Am Acad Dermatol. 2019;80:1073–1113.
- Augustin M et al. Acta Derm Venereol. 2010;90:147–151.
- Cohen AD et al. J Eur Acad Dermatol Venereol. 2009;23:561–565.
- Li WQ et al. Ann Rheum Dis. 2013;72:1200–1205.
- Cho JH. Nat Rev Immunol. 2008;8:458–466.
- Cottone M et al. Dig Dis. 2019;37:451–457.
- de Souza HSP, Fiocchi C. Nat Rev Gastroenterol Hepatol. 2016;13:13–27.
- Fujino S et al. Gut. 2003;52:65–70.
- Kurtovic NO et al. Med Arch. 2018;72:410–413.
- Alwan W, Nestle FO. Clin Exp Rheumatol. 2015;33(suppl 93):S2–S6.
- Mahil SK et al. Semin Immunopathol. 2016;38:11–27.
- Salas A et al. Nat Rev Gastroenterol Hepatic. 2020;17:323–337.
- Neurath MF. Nat Rev Immunol. 2014;14:329–342.
- Marafini I et al. Expert Opin Biol Ther. 2019;19:1207–1217.