more than a skin disease
References
- Mease PJ, Armstrong AW. Drugs. 2014;74:423–441.
- Armstrong AW, Read C. JAMA. 2020;323:1945–1960.
- Gottlieb AB, Merola JF. J Am Acad Dermatol. 2021;84:92–101.
- López-Medina C et al. RMD Open. 2021;7:e001450.
- Poddubnyy D et al. Semin Arthritis Rheum. 2021;51:880–887.
- Pittam B et al. Rheumatology (Oxford). 2020;59:2199–2206.
- Haroon M et al. Ann Rheum Dis. 2015;74:1045–1050.
- Carvahlo AL, Hedrich CM. Front Mol Biosci. 2021;8:662047.
- Elmets CA et al. J Am Acad Dermatol. 2019;80:1073–1113.
- Alinaghi F et al. J Am Acad Dermatol. 2019;80:251–265.e19.
- Merola JF et al. J Am Acad Dermatol. 2022;86:748–757.
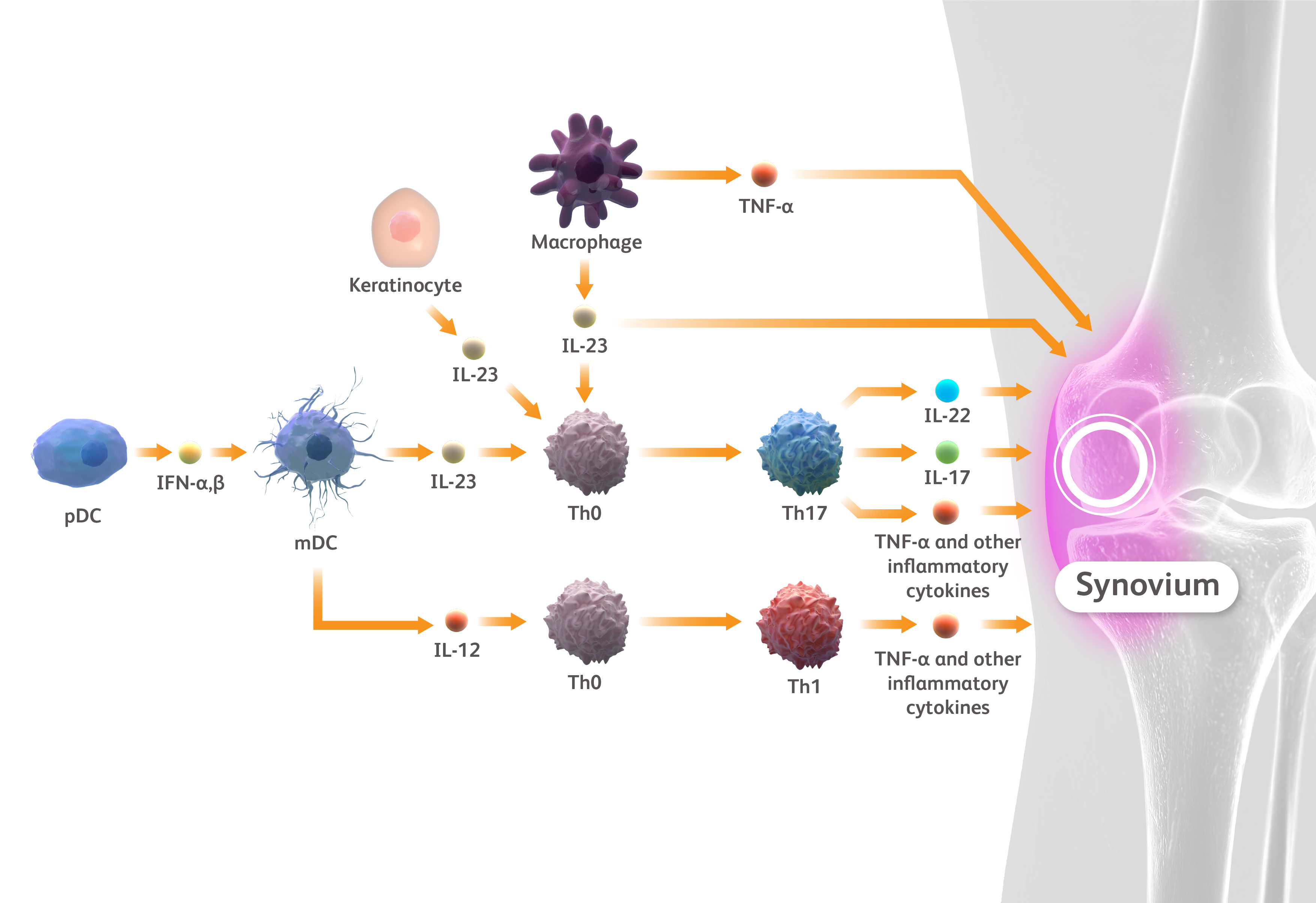
- Veale DJ, Fearon U. Lancet. 2018;391:2273–2284.
- Boutet MA et al. Int J Mol Sci. 2018;19:530.
- Ritchlin CT et al. N Engl J Med. 2017;376:957–970.
- Georgescu SR et al. Int J Mol Sci. 2019;20:739.
- Benham H et al. Arthritis Res Ther. 2013;15(5):R136. doi: 10.1186/ar4317.
- Leung YY et al. Front Med (Lausanne). 2018;5:246.
- Gottlieb AB et al. Dermatol Ther (Heidelb). 2021;11:1199–1216.
- Tom BDM et al. J Rheumatol. 2015;42:841–846.
- Husni ME et al. J Am Acad Dermatol. 2007;57:581–587.
- Helliwell PS. J Rheumatol. 2011;38:551–552.