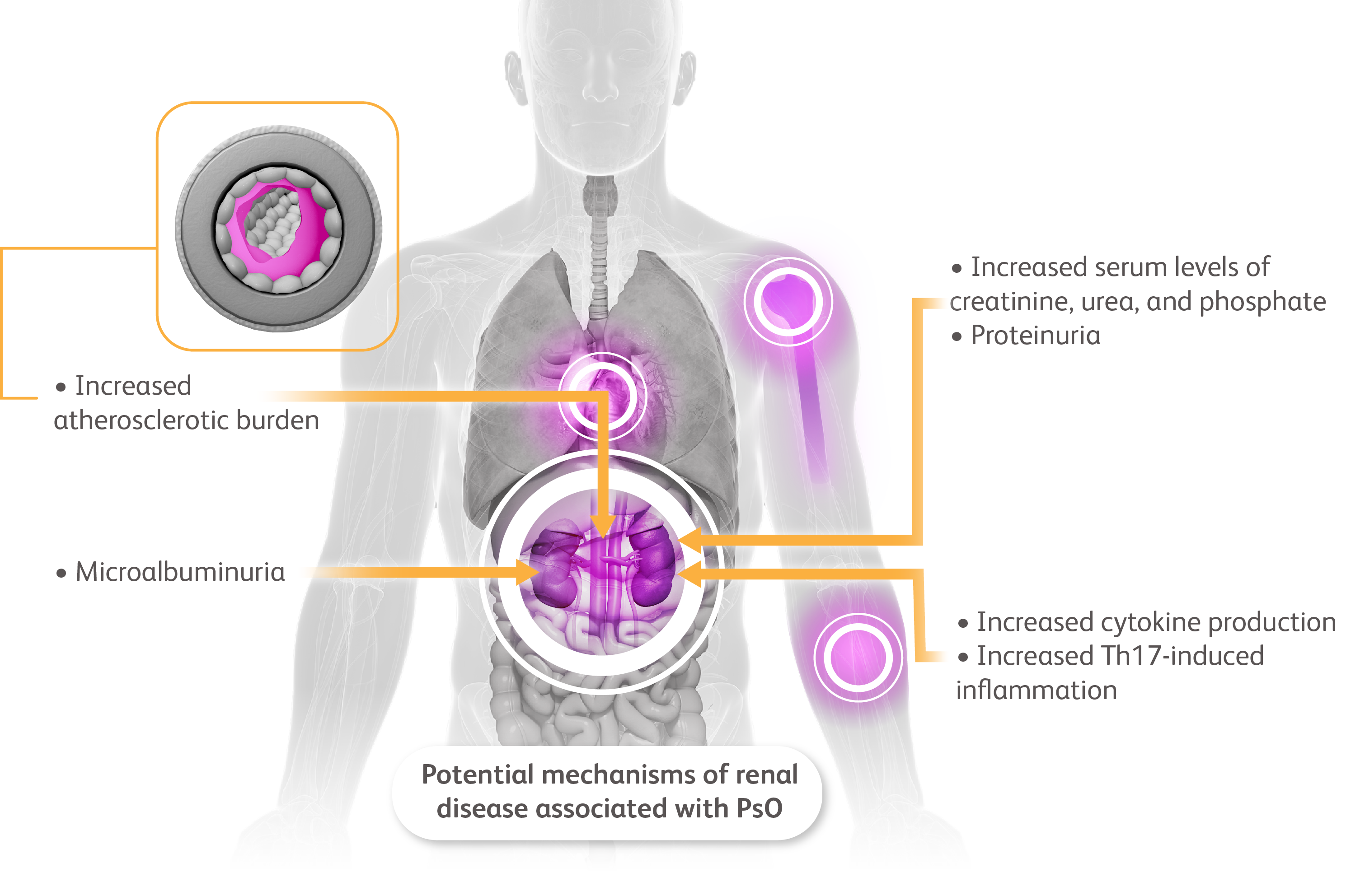
more than a skin disease
References
- González-Parra E et al. Actas Dermosifiliogr. 2016;107:823−829.
- Berl T, Henrich W. Clin J Am Soc Nephrol. 2006;1:8−18.
- Takeshita J et al. J Am Acad Dermatol. 2017;76:377−390.
- Ungprasert P, Raksasuk S. Int Urol Nephrol. 2018;50:1277−1283.
- Elmets CA et al. J Am Acad Dermatol. 2019;80:1073−1113.
- Tehranchinia Z et al. ScientificWorldJournal. 2018;2018:5301631.
- Szepietowski JC et al. J Eur Acad Dermatol Venereol. 2000;14:513−514.
- Cecchi R et al. Dermatology. 1992;185:93−95.
- Bilal J et al. Am J Med. 2018;131:1146−1154.
- Chi CC et al. J Dermatol Sci. 2015;78:232−238.
- Visconti L et al. Clin Rheumatol. 2016;35:297−302.
- Shahwan KT, Kimball AB. Med Clin North Am. 2015;99:1227−1242.
- Ogawa E et al. J Dermatol. 2018;45:264−272.
- Turner J-E et al. Kidney Int. 2010;77:1070−1075.
- Kitching AR, Holdsworth SR. J Am Soc Nephrol. 2011;22:235−238.
- Chiu H-Y et al. Br J Dermatol. 2015;173:146−154.